Modified versions of antibodies have been designed to improve certain properties or functionalities, e.g. to enhance their therapeutic or diagnostic potential.
Monoclonal antibodies (mAbs):
- Two identical binding sites each recognize the same target antigen.
- Monoclonal antibodies revolutionized medical therapeutics in the 1980s following the development of hybridoma technology. This technology enabled the production of antibodies that were identical in their antigen recognition, thereby providing a consistent therapeutic tool in various medical applications, notably in oncology and autoimmune diseases.
Bispecific antibodies (bsAbs):
- Two unique binding sites are directed at two different antigens or two different epitopes on the same antigen.
- Bispecific antibodies’ dual-targeting feature allows them to perform complex biological tasks such as linking cancer cells to immune cells to enhance cell destruction, a function not achievable with traditional mAbs. This functionality has been leveraged in recent therapeutic approaches, particularly in cancer immunotherapy.
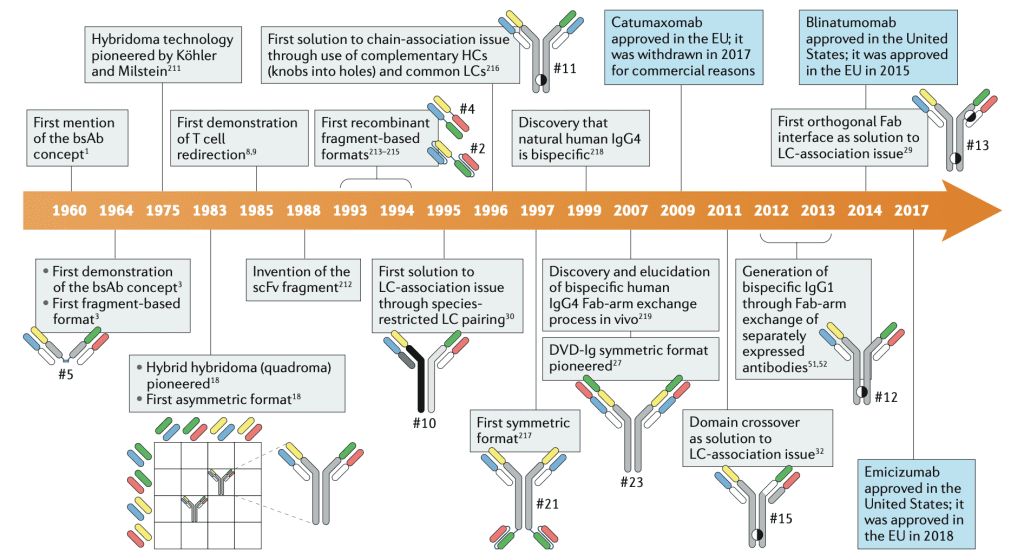
Historical Milestones in Antibody Engineering
1960s: The conceptual groundwork for bsAbs was laid. The first bispecific antibodies were described when Fabs from two polyclonal sera were re-associated into F(ab’)2 molecules after reduction and reoxidation.
1980s: The development of hybridoma technology facilitated the production of the first monoclonal Abs.
1992: The first article demonstrating the therapeutic potential of bsAbs was published, setting the stage for future clinical applications.
2000s: Catumaxomab and blinatumomab became the first bsAbs to receive approval for therapeutic use, validating the clinical efficacy of bsAbs in treating diseases.
Present: Ongoing advancements in antibody engineering continue to expand the therapeutic landscape, with newer antibody formats being explored for improved efficacy and safety profiles in clinical settings and further regulatory approvals of bsAbs.