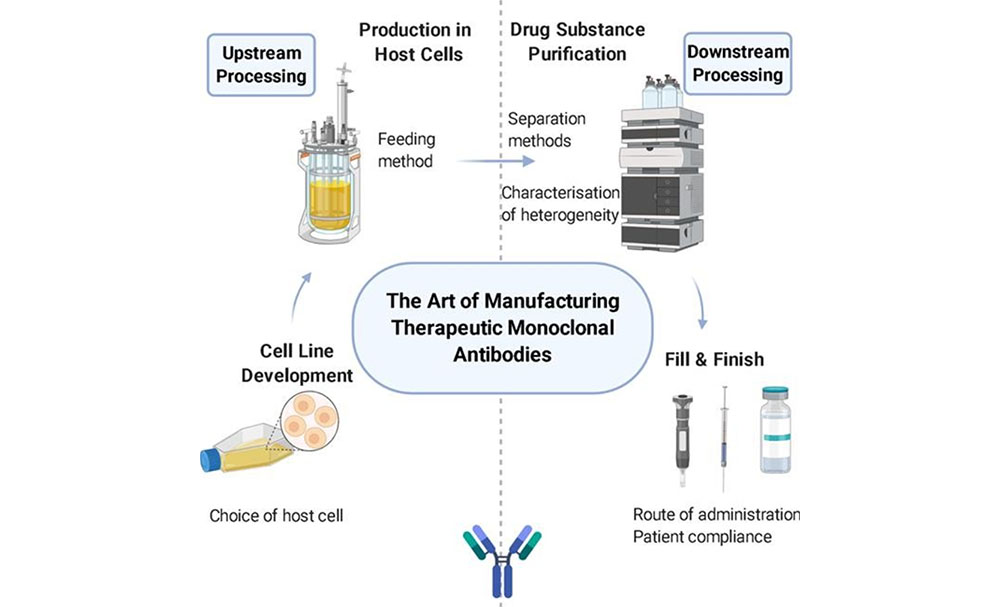
Therapeutic antibodies have revolutionized treatment paradigms across a range of diseases, including cancers, autoimmune disorders, and infectious diseases. Their production is a complex, multi-step process that begins with the development of specialized cell lines and extends through to careful optimization of growth conditions to maximize yield.
Cell Line Development
Among the host cells used for therapeutic antibody production, Chinese Hamster Ovary (CHO) cells are the most prevalent. CHO cells are favored for several reasons: they have a well-documented history of safe use in biopharmaceutical production, exhibit stable growth in suspension cultures, and are capable of producing high-fidelity, complex proteins that are therapeutically relevant. These cells can perform post-translational modifications, such as glycosylation, which are crucial for the biological activity and stability of antibodies. The adaptability of CHO cells to various bioreactor conditions also makes them ideal for large-scale production. Other host cells, like yeast cells or insect cells, are used but come with limitations in post-translational modification capabilities or lower yield, making CHO cells the industry standard for antibody production.
To produce therapeutic antibodies, host cells must first be genetically modified to express the desired antibody. This process involves the insertion of a gene encoding the antibody into the host cell’s DNA. Using techniques like transfection, scientists introduce a vector carrying the antibody gene into CHO cells. This vector includes not only the gene of interest but also selection markers to identify successfully modified cells and regulatory elements to ensure high-level expression of the antibody.
Once the gene is integrated into the cell’s genome, the cell line undergoes screening and selection to identify clones that exhibit high and stable antibody production. This step is critical, as variations in gene integration sites can lead to differences in expression levels. Advanced techniques, such as CRISPR/Cas9 and other gene-editing tools, have further refined the precision and efficiency of this genetic modification, enabling the targeted insertion of genes and the elimination of unwanted genetic elements that might downregulate antibody expression.
Furthermore, cell lines are often engineered to possess specific glycosylation patterns that enhance the therapeutic efficacy and reduce immunogenicity of the produced antibodies. For instance, modifying the cells to increase the proportion of certain glycosylation forms can improve an antibody’s ability to elicit immune responses (enhanced ADCC – Antibody-Dependent Cellular Cytotoxicity) or extend its half-life in circulation.1
Upstream Processing
The cultivation of genetically modified host cells is conducted in bioreactors, sophisticated vessels designed to support the optimal growth of cells under controlled conditions. Bioreactors range from small laboratory-scale units to large industrial-scale systems that can hold thousands of liters of culture. These systems provide a controlled environment where temperature, pH, agitation, and oxygen levels are meticulously regulated to meet the specific requirements of CHO cells.
In the initial phase, cells are cultured in small flasks and gradually adapted to the bioreactor’s environment. This scale-up process involves transferring the culture to progressively larger vessels, ensuring cells are acclimatized to their new conditions at each step. The bioreactors used for antibody production often employ a fed-batch culture method, where nutrients are periodically added to the culture to support cell growth and prolong the production phase. This method helps in achieving high cell densities and maximizing antibody yield.
Throughout the cultivation process, the condition of the cell culture is continuously monitored, with adjustments made as necessary to maintain optimal growth conditions. Advances in bioreactor design, such as the use of single-use bioreactors, have enhanced the flexibility and efficiency of the upstream process, reducing the risk of contamination and enabling faster turnaround times between production batches.2
Optimizing the conditions within the bioreactor is crucial for maximizing antibody production. Temperature, pH, and nutrient supply are among the key parameters adjusted to enhance cell growth and productivity. CHO cells typically thrive at temperatures around 37°C, but slight reductions in temperature can sometimes increase the stability and yield of the produced antibodies. The pH of the culture medium is carefully controlled, usually around 7.0, to ensure optimal cellular activity and product quality.
Nutrient supply is another critical factor; the culture medium must contain the right balance of amino acids, sugars, vitamins, and minerals to support cell growth and antibody production. In addition to the basal medium, feed strategies are developed to add nutrients and energy sources in a manner that prolongs the productive phase of the culture. These feeding strategies are tailored to the metabolic needs of the cell line and the kinetics of antibody production.
Oxygenation is also vital, as CHO cells require oxygen for their metabolic activities. Bioreactors are equipped with spargers and agitators to ensure sufficient oxygen transfer and to keep the cells in suspension. The oxygen level, along with agitation speed, is adjusted to meet the oxygen demand of the culture without causing shear stress that could damage the cells.3
The optimization of these conditions is a complex and iterative process, often involving the use of design of experiments (DoE) methodologies to identify the optimal set of conditions that lead to the highest productivity. Through careful control and adjustment of these parameters, bioprocess engineers can significantly enhance the yield and quality of therapeutic antibodies, ensuring their efficacy and safety for clinical use.
- Tihanyi, B., & Nyitray, L. (2020). Recent advances in CHO cell line development for recombinant protein production. Drug Discovery Today: Technologies, 38, 25-34. https://doi.org/10.1016/j.ddtec.2021.02.003
- Li, F., Vijayasankaran, N., Kiss, R., & Amanullah, A. (2010). Cell culture processes for monoclonal antibody production. MAbs, 2(5), 466-477. https://doi.org/10.4161/mabs.2.5.12720
- Liang, K., Luo, H., & Li, Q. (2023). Enhancing and stabilizing monoclonal antibody production by Chinese hamster ovary (CHO) cells with optimized perfusion culture strategies. Frontiers in Bioengineering and Biotechnology, 11, 1112349. https://doi.org/10.3389/fbioe.2023.1112349