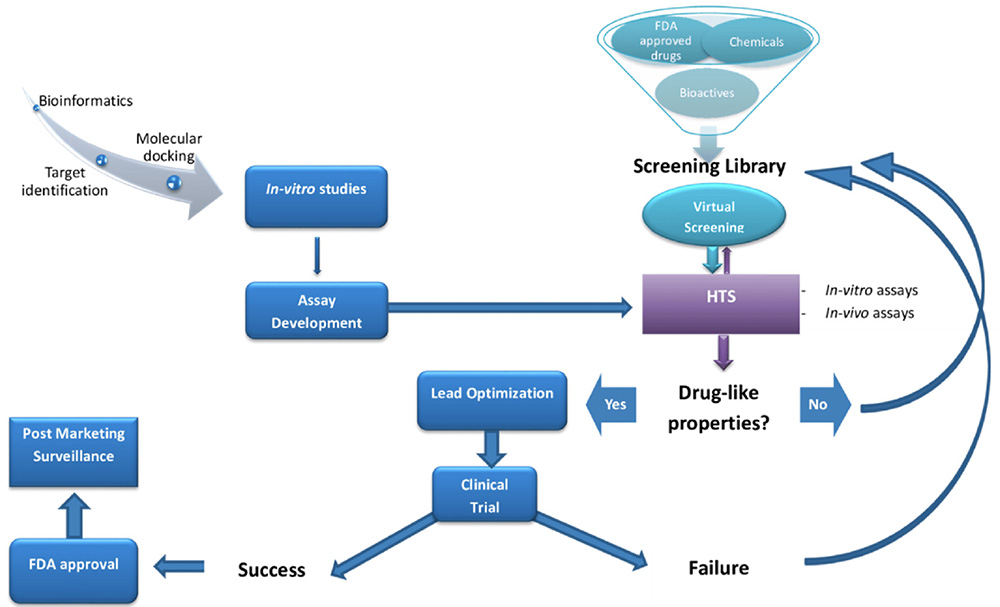
Once potential therapeutic antibodies are engineered, they undergo rigorous screening and selection to identify the most promising candidates.
Screening Methods
Traditionally, screening for potential therapeutic antibodies includes enzyme-linked immunosorbent assay (ELISA), a versatile tool measuring the binding affinity of antibodies to their target antigens. This provides valuable insights into the strength of their interaction. Surface plasmon resonance (SPR) takes it a step further by monitoring the real-time binding kinetics, offering crucial information about the speed and stability of the antibody-antigen interaction. Cell-based assays bridge the gap by assessing the antibodies’ biological activity in living cells, mimicking their real-world action against diseased cells or pathogens. Examples include cytotoxicity assays that evaluate cell-killing abilities and assays that measure immune cell activation. Finally, flow cytometry allows researchers to differentiate and analyze cell populations, enabling them to assess the impact of antibodies on specific cell types within a complex mixture.1
While these methods are invaluable, their traditional approach can be time-consuming and labor-intensive, especially when dealing with vast antibody libraries containing millions of potential candidates. This is where high-throughput screening (HTS) emerges by automating and miniaturizing screening processes. HTS allows researchers to test thousands of antibody variants simultaneously, significantly accelerating the identification of promising candidates. Examples of HTS technologies employed in antibody screening include:
- Phage display: Millions of antibody-presenting bacteriophages can be screened against immobilized antigens, rapidly identifying binders with desired characteristics.
- Microfluidic platforms: Miniaturized channels enable testing many antibody samples simultaneously with minimal reagent volumes, optimizing efficiency and speed.
- Bead-based assays: Antibodies are coupled to beads and exposed to various targets, allowing for multiplexed analysis and fast identification of specific binders, streamlining the selection process.2
Often, a combination of techniques is employed, with HTS guiding the initial selection and traditional methods providing in-depth characterization of promising candidates. As the field of antibody screening continues to evolve, innovative technologies like machine learning and artificial intelligence (AI) are being integrated to predict antibody properties and personalize screening strategies. This continuous advancement promises even more efficient and effective identification of next-generation therapeutic antibodies, ultimately translating into more targeted and successful treatments for various diseases.3
Criteria for Selection
- Specificity and Affinity: The antibody must exhibit high specificity for the target antigen and a strong binding affinity to ensure effective targeting and therapeutic action. However, for some therapeutic strategies, targeting broader categories of related antigens might be necessary for efficacy, requiring some flexibility in this criterion.
- Efficacy: Antibodies must demonstrate the ability to elicit the desired biological response, such as inhibiting disease-causing molecules or marking diseased cells for destruction by the immune system.
- Safety: Selected antibodies must show a low potential for adverse effects, such as triggering immune responses or cross-reactivity with non-target tissues.
- Developability: Considerations include the antibody’s stability, solubility, and manufacturability on a large scale without losing its functional properties.
The screening and selection phase narrows down the vast pool of engineered antibodies to the most promising candidates that combine high therapeutic potential with safety and manufacturability. As the field of antibody engineering continues to evolve, new selection strategies and sophisticated tools will undoubtedly emerge, further refining the process and paving the way for even more effective and targeted antibody-based therapies.
Biointron can help in your therapeutic antibody production. We provide services for HTP Recombinant Antibody Production, Bispecific Antibody Production, Large Scale Antibody Production, Afucosylated Antibody Expression, and RushMab™ – Small Scale Expression Packages. Learn more here: https://www.biointron.com/services/.
- Makowski, E. K., Wu, L., Gupta, P., & Tessier, P. M. (2021). Discovery-stage identification of drug-like antibodies using emerging experimental and computational methods. MAbs, 13(1). https://doi.org/10.1080/19420862.2021.1895540
- Sun, H., Hu, N., & Wang, J. (2022). Application of microfluidic technology in antibody screening. Biotechnology Journal, 17(8), 2100623. https://doi.org/10.1002/biot.202100623
- Kim, J., McFee, M., Fang, Q., Abdin, O., Kim, P. M. (2023). Computational and artificial intelligence-based methods for antibody development. Trends in Pharmacological Sciences, 14(3). https://doi.org/10.1016/j.tips.2022.12.005