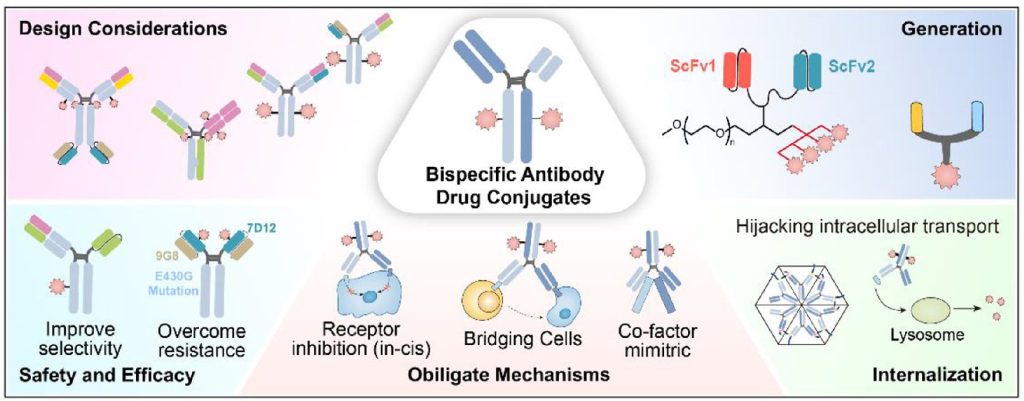

Bispecific antibody-drug conjugates (bsADCs) are an emerging class of therapeutics that combine the dual-targeting capabilities of bispecific antibodies (bsAbs) with the potent cytotoxic action of antibody-drug conjugates (ADCs). This innovative, next-generation ADC approach aims to enhance the selectivity and efficacy of cancer treatments, offering new hope in the fight against complex malignancies. As of January 2024, there are 10 bsADCs in clinical trials, but none are approved fully yet.1
Structure and Mechanism of Action
Bispecific antibodies have two different antigen-binding sites and are designed to target and bind to two distinct epitopes or antigens. ADCs are typically composed of a monoclonal antibody chemically linked to a cytotoxic payload. The mechanism of action of ADCs involves the specific binding to cancer cell antigens, followed by internalization of the conjugate into the cancer cells, where the linker is cleaved to release the cytotoxic drug. This process leads to targeted cell death, minimizing the side effects typically associated with chemotherapy.2
Despite notable successes in ADC development, significant challenges have emerged, including unexpected toxicity, limited improvements in toxicity profiles relative to the payload, and the development of resistance. These challenges are particularly pronounced in solid tumors due to several factors: the heterogeneous nature of solid tumors limits the efficacy of targeting a single antigen; therapeutic pressure can lead to antigen downregulation, epitope mutation, and activation of bypass pathways, resulting in ADC resistance; imprecise payload release due to factors like target antigen expression in normal tissues and linker instability can cause off-target toxicity; and resistance of the target antigen to internalization hinders a lethal effect. Therefore, bsADCs present themselves as a unique solution which can bind to co-expressed antigens in solid tumors to enhance selectivity, while also significantly improving internalization.1
Research Directions
Popular targets for BsADCs include HER2, EGFR, and c-MET. HER2 is an actionable and highly sensitive therapeutic target for the treatment of HER2-positive breast cancer, with various drugs, such as the monoclonal antibody trastuzumab, already approved globally.3 EGFR is a cell surface receptor that plays a crucial role in cell growth and proliferation but is often overexpressed or mutated in various types of cancer, including lung, colorectal, and head and neck cancers. c-Met is another cell surface receptor involved in cell growth, survival, and migration, with abnormal activation of c-Met signaling implicated in tumor progression, metastasis, and resistance to therapy in several types of cancer.
Designing the bsADC itself requires a holistic approach. Careful optimization is needed for the modular components of ADCs (the antibody, linker, and payload), as minor adjustments can significantly impact clinical outcomes. Swapping monoclonal antibodies with bispecific antibodies cannot comprehensively assess potential bsADCs either.
BsADCs represent just the beginning of promising developments in cancer therapy. Other next-generation strategies include ADCs featuring dual payloads, immune-modulating ADCs, radionuclide drug conjugates, masked ADCs, ADC combination therapy, and peptide-drug conjugates.1 The integration of innovative engineering techniques and a deeper understanding of tumor biology are likely to drive the future advancements of antibody therapeutics in oncology.
- Gu, Y., Wang, Z., & Wang, Y. (2024). Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B. https://doi.org/10.1016/j.apsb.2024.01.009
- Hong, Y., Nam, S. M., & Moon, A. (2023). Antibody-drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry. Archives of pharmacal research, 46(3), 131–148. https://doi.org/10.1007/s12272-023-01433-6
- Swain, S. M., Shastry, M., & Hamilton, E. (2023). Targeting HER2-positive breast cancer: Advances and future directions. Nature Reviews Drug Discovery, 22(2), 101-126. https://doi.org/10.1038/s41573-022-00579-0
