Antibody-based therapies provide highly specific treatments for various diseases. Among these, VHH antibodies, derived from camelids, have garnered significant attention due to their unique properties. Unlike conventional antibodies, which are composed of two heavy and two light chains, VHH antibodies consist of a single variable heavy chain domain. Previously, scientists viewed single-chain variable fragments (scFvs)—comprising VH and VL domains—as the smallest antibody fragments that could retain the same antigen-binding specificity as a full Ig molecule. However, the discovery of camelid VHH and shark variable new antigen receptors (VNARs) revealed that even a single V-like domain can maintain the affinity of a complete antibody molecule.1
Evolutionary Basis of VHH Antibodies in Camelids
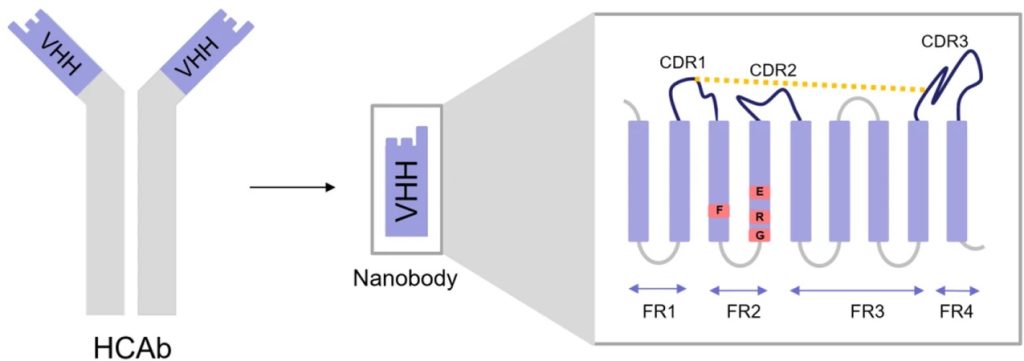
There are several hypotheses for the evolutionary emergence of VHHs, also known as heavy-chain antibodies (HCAbs). One of the most likely is that it provides an advantage due to their unique paratope structure, which is smaller and more elongated than conventional antibodies. This structure allows VHHs to access hidden epitopes, such as enzyme catalytic sites or viral cavities, more effectively. As a result, VHHs are frequently reported as enzyme inhibitors or virus neutralizers, suggesting they are well-suited for targeting conserved epitopes across different virus strains. The presence of non-canonical intradomain disulfide bridges enhances the stability of their long CDR3 loops and the overall VHH domain, enabling HCAbs to function under harsh physiological conditions.2
Most camelid HCAbs originate from specific IGHV genes that closely resemble the human VH3 family but differ in key areas, enabling function without a paired light chain. These differences include longer CDR-H3 loops, substitutions in the FWR2 region—shifting from hydrophobic to hydrophilic residues for better solubility—and non-canonical disulfide bonds. Although hallmark FWR2 substitutions are common, they aren’t essential for HCAb formation, as some antibodies from IGHV3 and IGHV4 families can form without them. Non-canonical disulfide bonds between CDR-H regions improve thermal stability and reduce entropic penalties from long CDR-H3s. Despite a large number of IGHV genes, the VHH repertoire in camelids is primarily derived from a few germlines, such as IGHV3-3 and IGHV3S53, often featuring hallmark residues or non-canonical cysteines.3
VHH Antibody Structure
Camelid VHHs, like the VH domain, consist of nine beta-strands forming an IgV fold, but the absence of the VL domain leads to significant differences, especially in the FR2 and hypervariable loops. In conventional VH regions, conserved hydrophobic amino acids facilitate the joining with VL. In contrast, VHHs replace these residues with more hydrophilic ones to avoid exposing the hydrophobic interface, thus enhancing solubility. The CDR3 domain of VHH folds over the hydrophobic interface to shield it. The hypervariable loops in VHHs extend to compensate for the absence of VL’s CDRs, offering a large antigen-interacting surface. Additionally, elongated CDR1 and CDR3 with mutational hotspots and extra disulfide bonds in VHHs enhance paratope diversity and provide flexibility in antigen binding, deviating from conventional antibody loop structures.1
Advantages of Camelid-Derived VHH Antibodies
The VHH domain is smaller than the variable regions of conventional antibodies, lacking the CH1 domain and light chains. The small size and single-domain structure of VHH antibodies enable them to access and bind cryptic epitopes that are often inaccessible to larger antibodies. This unique binding capability allows for more effective targeting of antigens, particularly in densely packed environments such as viral particles or intracellular proteins.
This structural simplification also provides VHH antibodies with enhanced stability and solubility, allowing them to maintain functionality under extreme conditions such as high temperatures and low pH. This makes them ideal for applications where traditional antibodies would denature or lose efficacy. Their high solubility further facilitates their use in various biochemical assays and therapeutic formulations. Because of their high thermostability, superior refolding capacity, and resistance to harsh conditions like the gastrointestinal tract, chemical denaturation, non-physiological pH, and high pressure. These qualities make VHHs ideal for applications in oral and aerosol therapies, antibody-drug conjugates, and integration into microchip biosensors.2
The simplicity of the VHH domain facilitates genetic manipulation, allowing for the creation of bispecific antibodies, fusion proteins, and other engineered constructs. This flexibility enhances the potential of VHH antibodies in both diagnostic and therapeutic applications, enabling the design of highly specific and multifunctional agents.
Applications of VHH Antibodies
VHH antibodies have been developed for a wide range of therapeutic applications, including cancer, infectious diseases, and inflammatory conditions. Their small size allows for rapid extravasation, deep tumor tissue penetration, and rapid tissue/blood clearance from the body, reducing the risk of off-target effects and toxicity.
In diagnostics, VHH antibodies are used for the detection of biomarkers in diseases such as cancer and infectious diseases. Their high specificity and stability make them ideal for use in various diagnostic platforms, including biosensors and imaging technologies.
VHH antibodies are invaluable tools in biomedical research, providing high-affinity reagents for studying protein-protein interactions, signaling pathways, and cellular processes. Their ability to bind to unique epitopes has expanded the toolkit available to researchers, enabling new insights into complex biological systems.
- Asaadi, Y., Jouneghani, F. F., Janani, S., & Rahbarizadeh, F. (2021). A comprehensive comparison between camelid nanobodies and single chain variable fragments. Biomarker Research, 9. https://doi.org/10.1186/s40364-021-00332-6
- Arbabi-Ghahroudi, M. (2022). Camelid Single-Domain Antibodies: Promises and Challenges as Lifesaving Treatments. International Journal of Molecular Sciences, 23(9). https://doi.org/10.3390/ijms23095009
- Bahrami Dizicheh, Z., Chen, I., & Koenig, P. (2023). VHH CDR-H3 conformation is determined by VH germline usage. Communications Biology, 6(1), 1-11. https://doi.org/10.1038/s42003-023-05241-y