What Are ADCs?
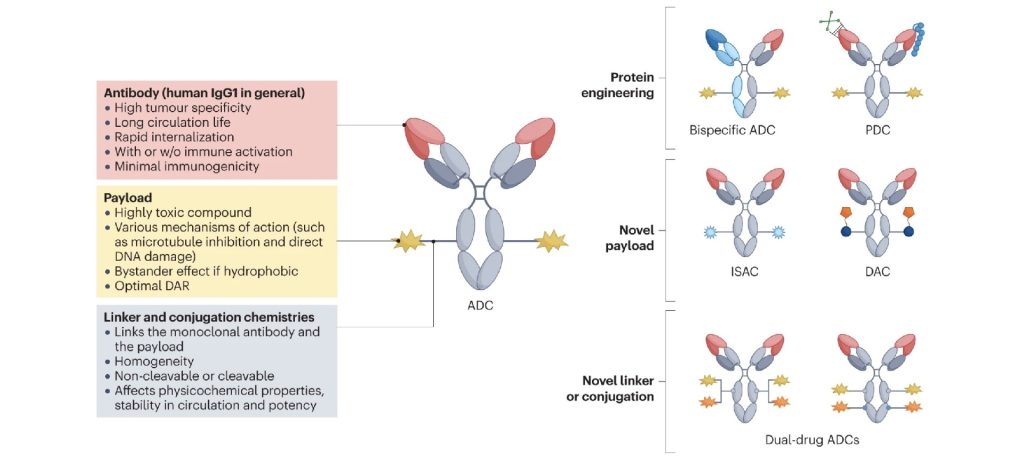
ADCs are complex biopharmaceuticals that consist of three key components:
- Monoclonal Antibody (mAb): This is the targeting component of the ADC, designed to bind to a specific antigen present on the surface of cancer cells.
- Cytotoxic Drug (Payload): Once the ADC binds to the cancer cell, the cytotoxic drug is released to kill the target cell. Common cytotoxins used include microtubule inhibitors and DNA-damaging agents.
- Linker: This is the chemical bond that attaches the cytotoxic drug to the antibody. The stability of the linker is crucial, as it must remain intact in the bloodstream but release the drug once the ADC reaches its target.
The precise engineering of these components determines the efficacy and safety of the ADC. Differences in the antibody, the drug, or the linker can lead to significant variations in the therapeutic outcomes.
ADC Biosimilars
ADC biosimilars are essentially “generic” versions of an existing, approved ADC drug (often referred to as the reference product). Like biosimilars in other therapeutic areas, ADC biosimilars are designed to be highly similar to their reference product in terms of structure, function, and clinical efficacy, but not identical.
Key characteristics include:
- Structural Similarity: The most critical aspect of an ADC biosimilar is its structural similarity to the reference ADC. This includes the monoclonal antibody, the cytotoxic payload, and the linker. However, minor differences in production processes can lead to variations in glycosylation patterns or other post-translational modifications.
- Regulatory Pathway: The development of ADC biosimilars involves rigorous testing to demonstrate biosimilarity. This includes comparative analytical studies, in vitro and in vivo functional assays, and clinical trials. The goal is to prove that any differences do not affect the ADC’s safety, efficacy, or immunogenicity.
- Market Impact: ADC biosimilars are introduced to the market after the patent protection of the reference product expires. They provide a more cost-effective alternative to the original ADC, potentially increasing patient access to these advanced therapies.
- Challenges in Development: Developing ADC biosimilars is complex due to the intricacies of their structure. The conjugation process, in particular, is challenging to replicate, making the production of biosimilars more demanding than that of traditional mAb biosimilars.
Structural characterization requires detailed analysis to ensure product consistency and functionality, which involves determining the drug-to-antibody ratio, attachment sites, potential by-products, and any free linker presence. Techniques like Circular Dichroism (CD), Fourier Transform Infrared Spectroscopy (FT-IR), Fluorescence, and Nuclear Magnetic Resonance (NMR) are essential for investigating the Higher Order Structure (HOS) of ADCs. These methods help assess potential structural alterations caused by conjugation, which may impact the ADC’s efficacy and safety. Aggregation studies, using orthogonal techniques, are also crucial to identify any structural disruptions.1
ADC Isotypes
ADC isotypes, on the other hand, refer to ADCs that share the same antigen-binding region but differ in their constant region, or Fc region, of the antibody. This difference in the Fc region can have significant implications for the ADC’s pharmacokinetics, pharmacodynamics, and overall therapeutic profile.
Key characteristics include:
- Structural Differences: Unlike biosimilars, ADC isotypes are not intended to be identical or even similar to another ADC in totality. Instead, they are designed to explore different therapeutic properties by varying the antibody isotype. For example, switching from an IgG1 to an IgG2 isotype can reduce the Fc-mediated effector functions, which might be desirable in certain therapeutic contexts.
- Therapeutic Implications: The choice of antibody isotype in an ADC can influence its distribution, half-life, and ability to elicit immune responses. For instance, IgG1 isotypes are typically more effective at mediating antibody-dependent cellular cytotoxicity (ADCC), while IgG2 isotypes may be preferable for applications where reduced effector function is desired.
- Development and Application: ADC isotypes are often developed as part of a strategy to optimize the therapeutic profile of an ADC for specific clinical indications. They are not copies of existing ADCs but are instead new entities with distinct properties that may offer advantages in particular cancer types or patient populations.
- Regulatory Considerations: Since ADC isotypes represent new molecules, they undergo the full regulatory approval process as novel drugs. This involves extensive preclinical and clinical testing to ensure their safety and efficacy.
Ultimately, the purpose of ADC biosimilars are to offer a cost-effective alternative to existing ADCs, whereas ADC isotypes are developed to explore and optimize different therapeutic properties. ADC biosimilars follow a streamlined regulatory pathway that focuses on demonstrating similarity to an existing product, whereas ADC isotypes would undergo full regulatory approval as new drugs.